The FDA clears updated COVID-19 vaccines for kids under age 5 : NPR

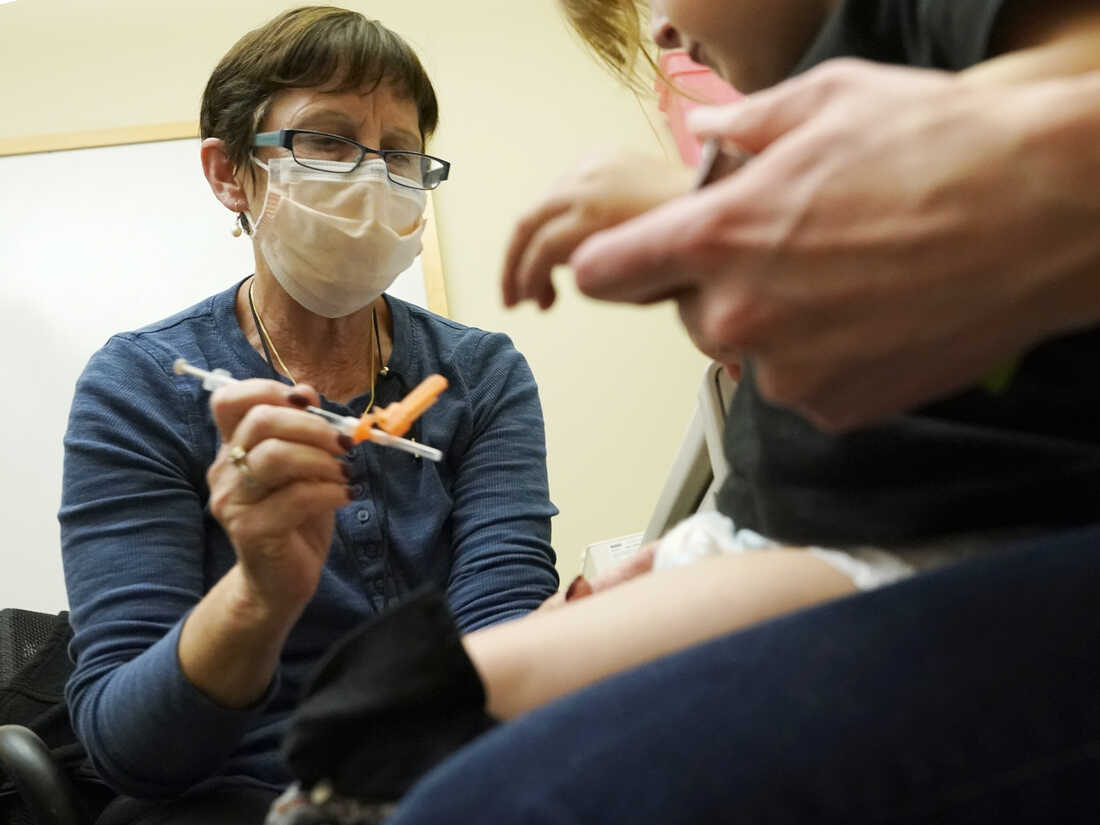
A nurse at the University of Washington Medical Center clinic in Seattle administers Pfizer’s COVID-19 vaccine to a 20-month-old child on June 21.
Ted S. Warren/AP
hide captions
switch captions
Ted S. Warren/AP
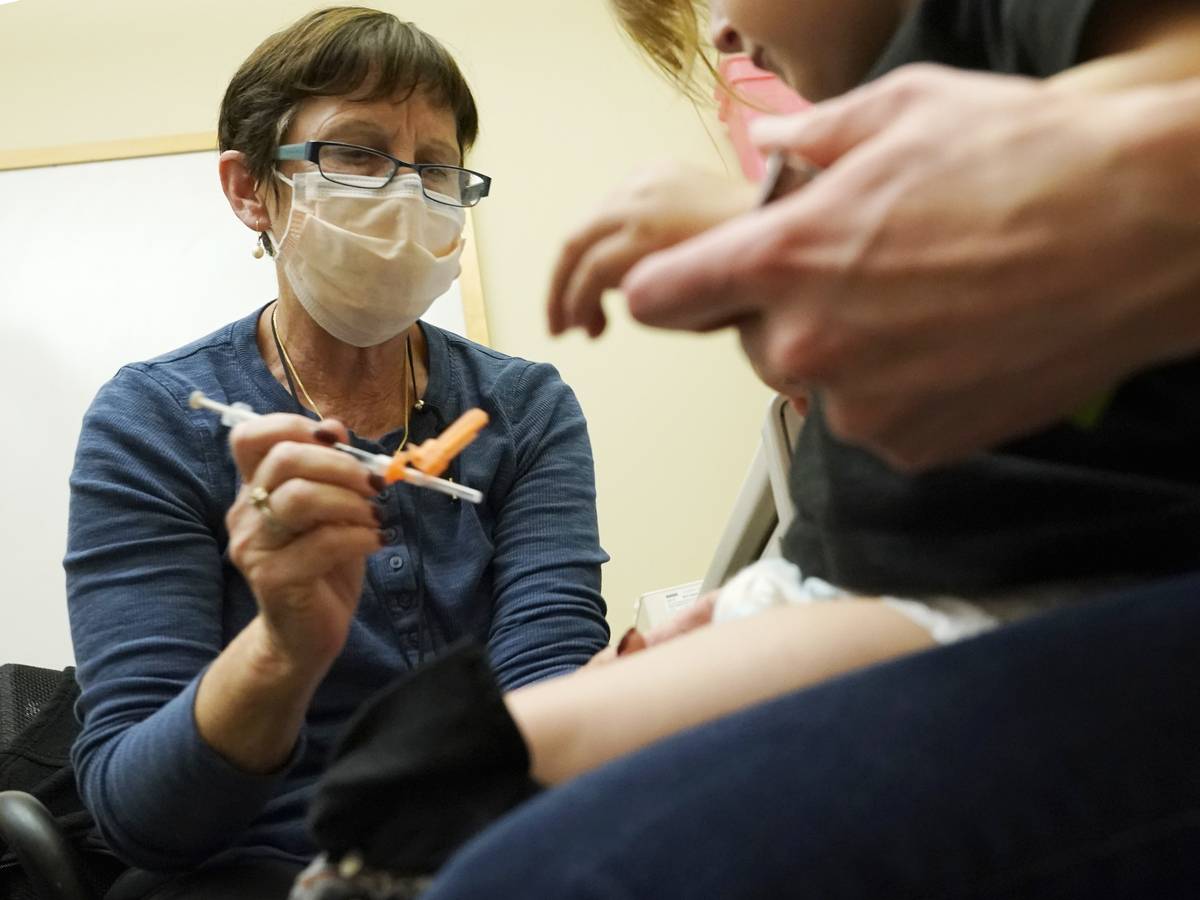
A nurse at the University of Washington Medical Center clinic in Seattle administers Pfizer’s COVID-19 vaccine to a 20-month-old child on June 21.
Ted S. Warren/AP
US regulators on Thursday removed an updated dose of the COVID-19 vaccine for children under the age of 5.
The Food and Drug Administration’s decision is aimed at better protecting the youngest children amid a growing number of COVID-19 cases across the country — at a time when children’s hospitals I was filled with children with other respiratory illnesses, including the flu.
Dr Peter Marks, the FDA’s director of vaccines, told The Associated Press: “Immunization is the best way we know to help prevent serious consequences of COVID-19, such as hospitalization and death. “.
Omicron-targeted booster shots made by Moderna and rival Pfizer are available to everyone 5 years of age and older.
The FDA now allows modified shots to be used starting at 6 months of age – but who is eligible depends on how many vaccines they’ve had and what type. Only about 5% of children under the age of 5 have received all of their primary immunizations since vaccinations for the youngest began in June.
The FDA has decided that:
–Children under 6 years of age who have received two initial doses of Moderna’s COVID-19 vaccine can receive a single booster dose of Moderna’s updated formulation if it has been at least two months since the last shot.
–Pfizer’s vaccine requires three initial doses for children under 5 years of age — and those who have not completed that series will receive the original formulation for the first two shots and an omicron-targeted version for third injection.
The Centers for Disease Control and Prevention is expected to sign off soon, the final step to starting vaccinations.
Marks said the bivalent vaccine is safe for young children and will help parents “maintain protection for those children in the most up-to-date way possible.”
However, children under 5 years of age who have received all three doses of Pfizer are not eligible for a booster shot.
For now, “the good news is that they can be pretty well protected,” says Marks.
The FDA expects data from Pfizer and its BioNTech partner sometime next month to determine if those children need an omicron-targeted booster “and we’ll act as soon as we can.” he said.
For parents who haven’t vaccinated their kids, it’s not too late — especially as “we’re entering a period when COVID-19 cases are on the rise,” says Marks.
Moderna and Pfizer’s updated vaccines are combination shots, containing half the original vaccine and half modified to accommodate the omicron strains BA.4 and BA.5 that until recently remained have advantages. Now BA.5 descendants are responsible for most of the COVID-19 cases.
The CDC last month released the first real data showing that an updated booster, using either company’s version, would provide additional protection for adults. The analysis showed the greatest benefit was that in those who had never received a booster before, only two initial doses of the COVID-19 vaccine were needed – but even those who received a dose in the summer were more protected than with them skipping that dose. latest shot.







