Promising drug for Mpox fails trial as virus spreads in Africa
As mpox continues ARRIVE spread in Central AfricaA promising antiviral drug to treat the infection failed to improve patients’ symptoms in a trial in the Democratic Republic of Congo, the epicenter of the outbreak.
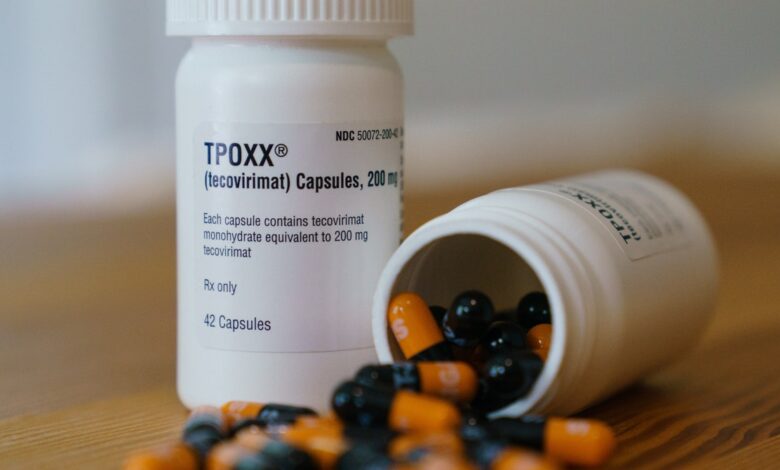
In trials, the drug tecovirimat, also known as TPOXX, did not reduce the characteristic blister-like rash in people with mpoxformerly known as monkeypox. In an unusual move, the US National Institute of Allergy and Infectious Diseases (NIAID), which funded the study, released the initial findings earlier this month before the full results are peer-reviewed and published in scientific journals.
Lori Dodd, director of NIAID’s clinical trials research and statistics branch, told WIRED that the agency shared the initial results “due to the urgent need for scientific evidence supporting the use of tecovirimat for the treatment of mpox.” That urgency, she said, was reinforced when the World Health Organization designated the outbreak of mpox in Central Africa as a global health emergency on August 14. This is the second such announcement in two years.
The results are disappointing, especially as central African countries struggle to contain the spread of mpox. Since the beginning of the year, 13 African countries have recorded a total of 20,720 confirmed or suspected cases of mpox and 582 deaths, according to a Report August 25 from the Africa Centers for Disease Control and Prevention.
On Monday, the World Health Organization has launched a six-month strategic plan The plan, estimated to cost $135 million, includes strengthening surveillance efforts and improving access to testing and vaccines. “Mpox outbreaks in the Democratic Republic of the Congo and neighboring countries can be controlled and contained,” Tedros Adhanom Ghebreyesus, WHO Director-General, said in a statement.
There are vaccines approved to prevent mpox but no medications are indicated to treat the disease. Tecovirimat is Approved by the US Food and Drug Administration in 2018 to treat smallpox, a related virus, and ongoing trials of the drug have been launched in 2022 to treat smallpox amid a global outbreak. The drug is also available in the United States to treat smallpox through an expanded access program, which allows doctors to treat patients with the disease. drug under study outside clinical trials. In the UK and Europe, TPOXX has been approved for mpox in special cases without comprehensive data on its efficacy.
As part of the DRC trial, nearly 600 participants were randomly assigned to receive either tecovirimat or a placebo and were hospitalized for at least 14 days, where they were closely monitored. All participants received supportive care, including nutrition, hydration, and treatment of any secondary infections. While the drug was found to be safe, it was no better at clearing patients’ lesions than the placebo.
Notably, the mortality rate was lower and the patients’ lesions recovered more quickly than expected regardless of whether they received tecovirimat or placebo. The study’s overall mortality rate of 1.7 percent among those enrolled, regardless of whether they received the drug, was much lower than the mpox mortality rate of 3.6 percent or more reported among all cases in the DRC.