Book Review: Mildred Dresselhaus’s Research Proved We’re All Wrong About Graphite

Mildred Dresselhaus’s life was one that defied everything. Growing up poor in the Bronx – and even to her detriment, growing up as a woman in the 1940s – Dresselhaus’s traditional career options were bland. Instead, she rose to become one of the world’s leading experts in carbon science as well as the first female Institute Professor at MIT, where she spent 57 years of her career. She has collaborated with physical scientists such as Enrico Fermi and laid the essential foundation for future Nobel Prize-winning research, directed the Office of Science at the US Department of Energy and was awarded the Medal herself. National Science.
In the below excerpt from The carbon queen: The remarkable life of nanoscience pioneer Mildred Dresselhaus, Author and Deputy Editor-in-Chief at MIT . NewsMaia Weinstock, tells of the time Dresselhaus teamed up with Iranian-American physicist Ali Javan to investigate exactly how charge carriers – i.e. electrons – move in a graphite matrix, research that will completely overturn understanding knowledge of the field about how these subatomic particles operate.

MIT Press
Taken from The carbon queen: The remarkable life of nanoscience pioneer Mildred Dresselhaus by Maia Weinstock. Reprinted with permission of The MIT Press. Copyright 2022.
ONE STANDARD SPACE
For anyone with a research career as long and as accomplished as Mildred S. Dresselhaus, there are bound to be certain papers that can get a little lost in the corridors of the mind — articles that just get there. moderate strides, perhaps, or that involve relatively little effort or input (for example, when as a small consulting author on a paper with multiple co-authors). On the contrary, there are always outstanding articles that one can never forget — for their scientific impact, to coincide with particularly memorable periods in one’s career, or simply Unique or interesting experiments.
Millie’s first major research publication after becoming a permanent member of the MIT faculty is outstanding. It is a story she has described many times in her career recollections, calling it “an interesting story for the history of science.”
The story begins with a collaboration between Millie and Iranian-American physicist Ali Javan. Born in Iran to Azerbaijani parents, Javan is a gifted scientist and award-winning engineer who is best known for inventing the gas laser. His helium-neon laser, co-invented with William Bennett Jr. while they were both at Bell Labs, was a step forward that helped create many of the most important technologies of the late twentieth century — from CD and DVD players to bar code scanning systems to modern fiber optics. .
After publishing a few papers describing her initial magneto-optical research on the electronic structure of graphite, Millie was looking to delve further and Javan wanted to help. The two met during Millie’s work at Lincoln Laboratories; she is a huge fan, once calling him “a genius” and “an extremely creative and brilliant scientist”.
For her new work, Millie aimed to study the magnetic energy levels in the valence and conduction bands of graphite. To do this, she, Javan, and a graduate student, Paul Schroeder, used a neon gas laser that would provide a sharp point of light to probe their graphite samples. Lasers had to be specially made for the experiment, and it took years for the fruits of their labor to mature; actually, Millie moved from Lincoln to MIT in the middle of a job.
If the experiment just turned out to be the same as everything the team already knew, it would still be a road-breaking exercise because it was one of the first experiments that scientists used lasers to study. the behavior of electrons in a magnetic field. But the result was not pleasant at all. Three years after Millie and her colleagues began experimenting, they discovered their data was telling them what seemed impossible: the energy-level gap in the valence and conduction bands of perfect graphite completely different from what they expected. As Millie explained to an avid audience at MIT two decades later, this meant that “the band structure that people had used up to that point certainly couldn’t be right and had to be turned upside down.”
In other words, Millie and her colleagues are about to overturn a well-established scientific rule — one of the more interesting and important kinds of scientific discoveries one can make. Just like the landmark 1957 publication led by Chien-Shiung Wu, who overturned a long-accepted particle physics concept known as conservation of parity, changing science to Setting requires a high degree of accuracy — and confidence in one’s results. Millie and her team had both.
Their data suggest that the previously accepted positions of so-called charge carriers in the electronic structure of graphite are indeed out of date. Charge carriers, which allow energy to flow through a conductive material like graphite, are essentially what their name suggests: something that can carry an electrical charge. They are also important for the operation of electronic devices powered by an energy flow.
Electron is a well known charge carrier; these subatomic bits carry a negative charge as they move around. Another type of charge carrier can be seen when an electron moves from atom to atom in a crystal lattice, creating something in empty space that also carries a charge — a particle the size of electrons but opposite charge. Essentially lacking electrons, these positively charged particles are called holes.
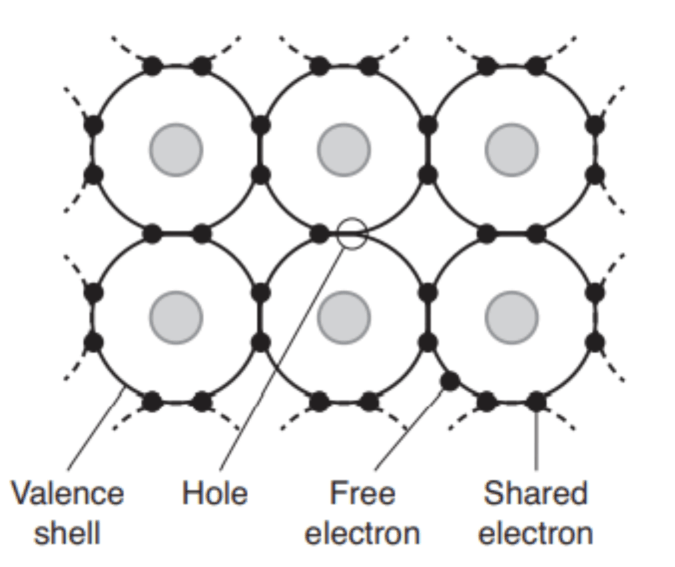
MIT Press
FIGURE 6.1 In this simple diagram, electrons (black dots) surround the nucleus of an atom in a crystal lattice. In some cases, electrons can burst out of the crystal lattice, leaving a positively charged spot or hole. Both electrons and holes can move, affecting electrical conduction within the material.
Millie, Javan and Schroeder discovered that scientists were using the wrong way of assigning holes and electrons in the previously accepted structure of graphite: they found electrons in the hole positions, and vice versa. . “This is pretty crazy,” Millie said in a 2001 historical interview. “We discovered that everything that had been done on the electronic structure of graphite up to that point was reversed. .”
As with many other discoveries that upset conventional wisdom, the acceptance of revelation was not immediate. First, the journal to which Millie and her collaborators submitted their paper initially refused to publish it. In recounting the story, Millie often noted that one of the referees, her friend and colleague Joel McClure, had privately revealed herself as a reviewer in hopes of convincing her that she had lost ground a shameful way. “He said,” Millie recalled in a 2001 interview, “,” Millie, you don’t want to publish this. We know where the electrons and holes are; How can you say they are backwards? ‘”But like all good scientists, Millie and her colleagues have checked and rechecked their results over and over and are confident in their accuracy. And so Millie thanked McClure and told him they believed they were right. Millie recounted in 1987: “We wanted to publish, and we … would risk ruining our careers.
Giving their colleagues the benefit of the doubt, McClure and other peer reviewers approved publication of the paper despite conclusions that contradicted the established structure of graphite. Then something funny happened: reinforced by seeing these conclusions in print, other researchers came out with previously collected data that only made sense when assigned. inversion of electrons and holes. Millie said in 2001: “There are many publications supporting our discovery that were previously inexplicable.
Today, those who study the electronic structure of graphite do so with the knowledge of carrier positions gathered by Millie, Ali Javan, and Paul Schroeder (who ended with a rather remarkable thesis). attention based on group results). For Millie, who published work during her first year at the MIT faculty, the experiment quickly cemented her status as a distinguished Institute researcher. Although many of her most notable contributions to science have yet to be published, this initial discovery is one she will remain proud of for the rest of her life.
All products recommended by Engadget are handpicked by our editorial team, independent of our parent company. Some of our stories include affiliate links. If you buy something through one of these links, we may earn an affiliate commission.



